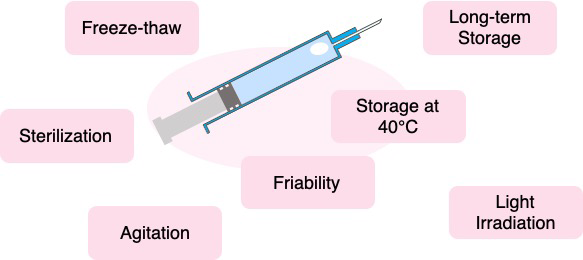
Stability Testing
Biopharmaceuticals are subjected to various stresses during manufacturing, transportation, storage, and administration. It is essential to evaluate the stability of the product in its container. We use a variety of methods to evaluate the impact of various stresses on the drug product in its container.
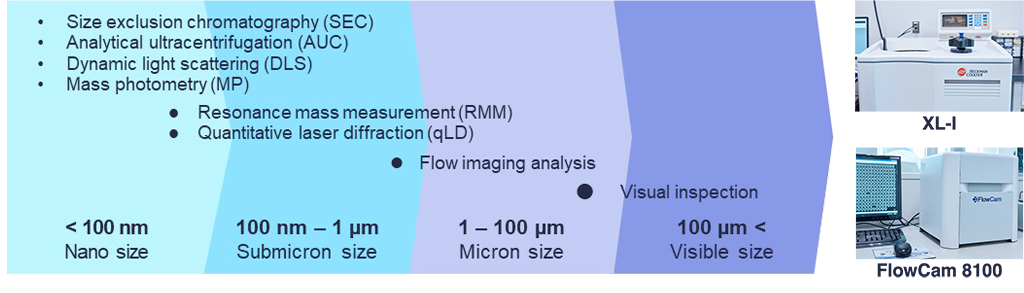
Quanifitation of Protein Aggregates
The size of aggregates of biopharmaceuticals ranges from tens of nanometers to hundreds of micrometers. We analyze the concentration and size distribution of aggregates using a variety of analytical techniques.
Characterization for Protein Aggregates
LC-MS, native MS, and SDS-PAGE are used to analyze aggregate components.
Structure Analysis
Post-translational modifications such as oxidation, disulfide, and deamidation are evaluated by peptide mapping using LC-MS/MS.
Quantification of Protein Adsorption on the Surface of Containers
The amount of protein adsorbed on the surface of vials and syringes is quantified using the ultra performance LC (UPLC) and micro BCA methods.
Immunogenicity Assessment
Protein aggregates and silicone oil droplets are considered a potential source of immunogenicity. By monitoring the secretion of innate immune system cytokines from human peripheral blood mononuclear cells, we can evaluate the evoked immune response.
Insoluble Particle Analysis
Micron-sized aggregates can be analyzed by flow imaging analysis. FlowCam provides information on the size, number, and shape of particles by continuously capturing high-resolution images of particles in a liquid as they pass through a glass flow cell.

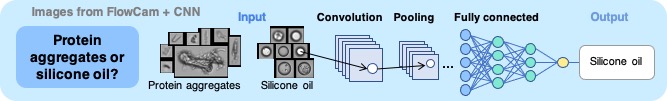
Example of measurement of micron particles in antibody drugs
Quantification of Silicone Oil Droplets
Some pharmaceuticals filled in pre-filled syringes may contain silicone oil droplets. Images obtained by FlowCam can be classified into protein aggregates and silicone oil droplets using convolutional neural networks (CNN).