Quality Analysis and Manufacturing Services for Gene Therapy
Methods of gene delivery include the iin vivo method, which involves directly administering genes into the body using viral vectors, and the ex vivo method, which involves culturing cells with introduced genes outside the body before transplanting them back into the body. In both methods, viral vectors play a crucial role as carriers for the genes.
You Medico provides high-precision quality analysis services for adeno-associated virus vectors (AAV), lentivirus, and oncolytic viruses. We are equipped for BSL-2 and P2 facilities, and plan to launch GMP-compliant analysis services in fiscal year 2025.
Additionally, leveraging our expertise in analytical technology, we offer AAV manufacturing services with clearly guaranteed quality.
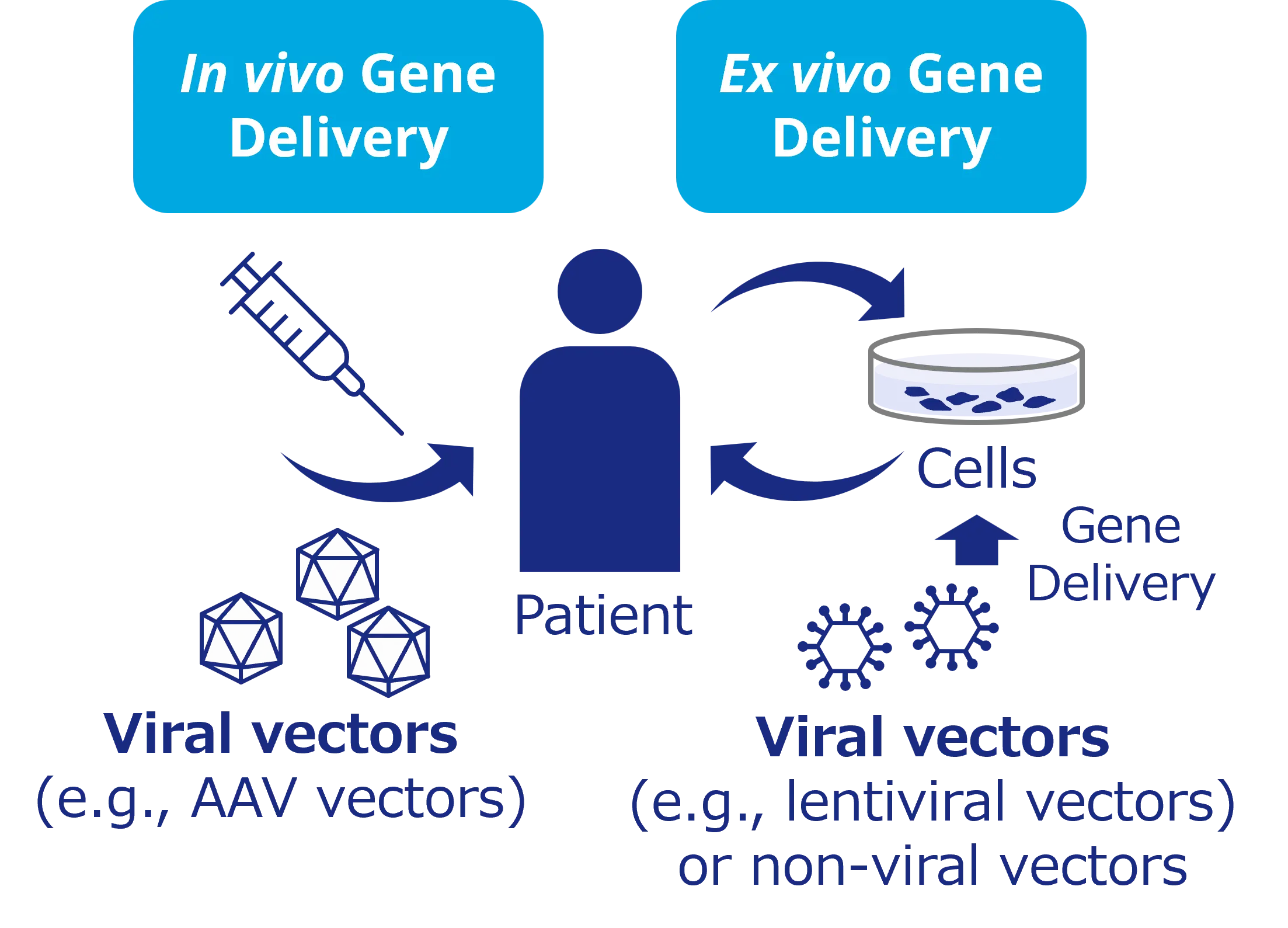
Overview of Gene Therapy
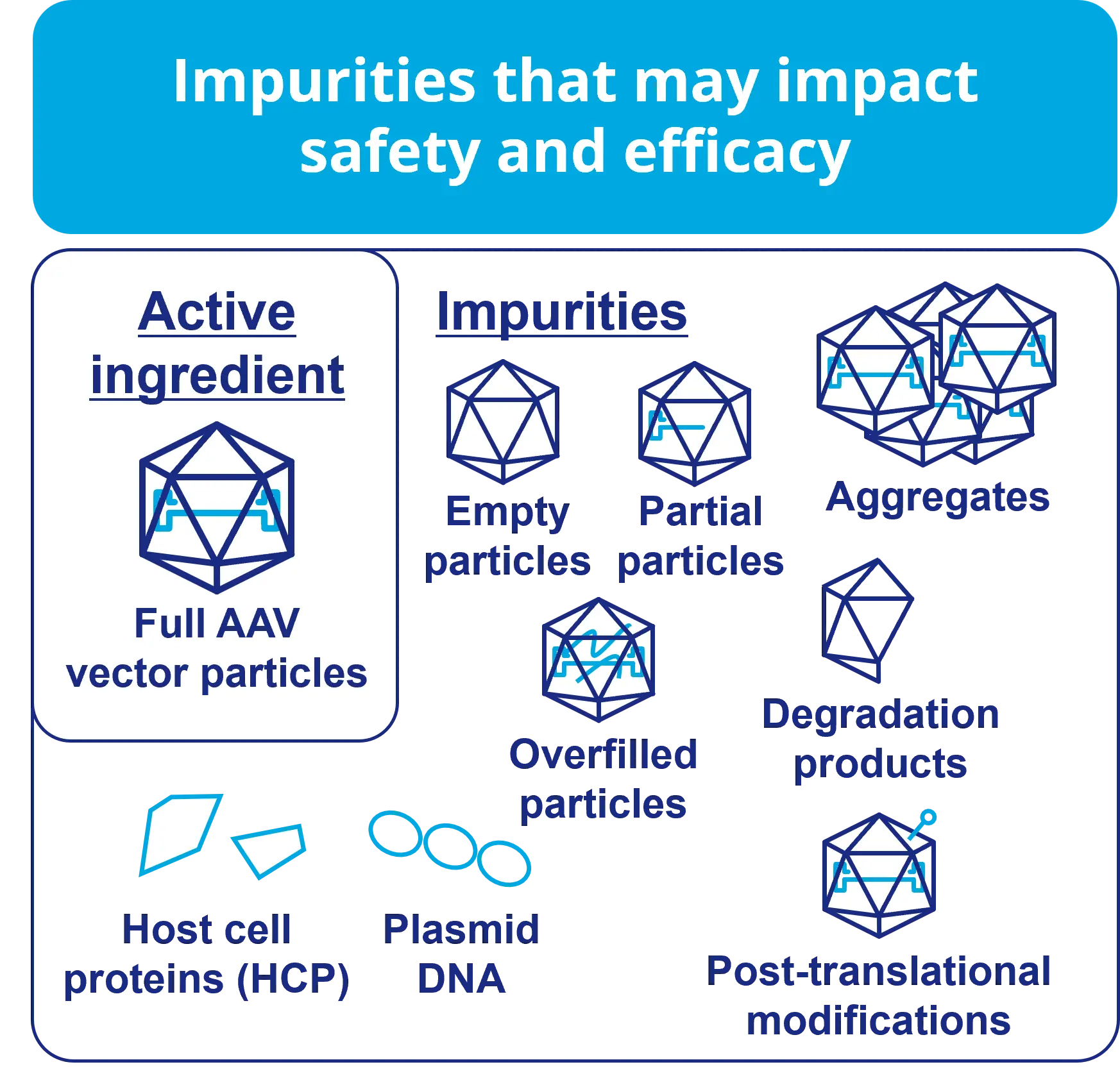
impurities that may impact safety and efficacy
Quality Analysis
AAV Vector Manufacturing and Sales
FAQ

Is it possible to determine the ratio of full particles to empty particles in an AAV vector?

Yes, it is possible. We offer analytical methods tailored to each development stage and sample volume, including ultracentrifugation analysis (AUC), recognized as the gold standard for AAV vector dispersion analysis.
The management of empty particles, which are impurities in AAV vectors, is required by FDA draft guidance due to their potential to cause immunogenicity. Furthermore, quantification is required not only for empty particles but also for intermediate particles and overfill particles that could become impurities derived from the target substance. AUC and CD-MS are recommended as analytical methods capable of performing this quantification.
Link:Full / Empty Particle Ratio
Size Distribution Analysis of the Adeno-Associated Virus Vector by the c(s) Analysis of Band Sedimentation Analytical Ultracentrifugation with Multiwavelength Detection, Maruno et al. J Pharm Sci. 2023 Apr;112(4):937-946.
Multimass Analysis of Adeno-Associated Virus Vectors by Orbitrap-Based Charge Detection Mass Spectrometry, Nakatsuka et al. Anal Chem. 2024 Oct 22;96(42):17037-17046.

Is it possible to quantify the ratio of intact particles to empty particles for unpurified AAV vectors in culture medium or cell lysate?

Yes, it is possible. We have established an analytical method to distinguish between intact and empty particles of unpurified AAV vectors and directly quantify the intact particle ratio and genomic titer using mass spectrometry.
Link:Full / Empty Particle Ratio
Direct Identification and Quantification of Recombinant Adeno-Associated Virus in Crude Cell Lysate and Conditioned Medium by Mass Photometry, Yamaguchi et al. Anal Chem. 2025 May 20;97(19):10405-10416.

We would like to perform performance evaluations of analytical instruments, manufacturing equipment, and purification systems, as well as analytical SSTs. Are there any AAV vectors with well-defined characteristics?

We manufacture and sell high-purity, high-quality AAV vector reference materials in-house, essential for gene therapy research. These materials are fully characterized for key quality attributes, including complete particle ratio, titer, and purity, determined by ultracentrifugation analysis.
Link:AAV Vector Reference Product

Regarding the viral vector currently under development, could you advise which characteristic analysis items should be performed and what type of analysis should be used?

We have extensive experience analyzing diverse viral vectors and viruses, including AAV vectors, retroviruses, lentiviruses, measles virus, and Sendai virus. Based on this expertise, we can advise you on the specific characterization analyses required for your viral vectors under development and recommend appropriate analytical methods. We can also perform these analyses for you. Furthermore, incorporating advice from our CSO, Uchiyama, who serves as an expert committee member for the United States Pharmacopeia (USP), we provide proposals that take regulatory requirements into account.

Does it support P2 and BSL2?

We support it.

During stability testing of biopharmaceuticals and gene therapy products, insoluble particulate matter was detected. Could you propose tests to identify the cause and resolve this issue?

We have extensive experience in identifying the causes of insoluble particulate matter generation and implementing suppression measures, having successfully resolved numerous client requests. We tailor our approach to each client's specific case, proposing analytical methods and recommending stable formulations that minimize the generation of insoluble particulate matter.

Can you also provide support for regulatory consultations and preparation of application documents related to CMC, in addition to contract analysis?

Yes. In addition to contract analysis, we can provide application-related support such as attending PMDA consultations and preparing and advising on CMC-related documents submitted to regulatory authorities.

Is it possible to evaluate whether containers or container materials under development can be used for pharmaceuticals?

The evaluation of pharmaceutical suitability for containers and container materials such as syringes, vials, and IV bags can be comprehensively conducted, from test design to the acquisition of necessary data.

Is it possible to evaluate the adsorption of pharmaceuticals onto containers?

It is possible to quantitatively evaluate the adsorption amount.

I want to analyze modifications such as oxidation, deamidation, and phosphorylation of proteins.

Peptide mapping using mass spectrometry enables the analysis of post-translational modifications and chemical modifications in proteins, such as oxidation, deamidation, and phosphorylation. We will determine the modification sites and report the modification rates at each site.
Analysis of antibodies, viral vectors, and viruses is possible.

Can AUC be used for QC and shipping tests of clinical samples?

Yes, it is possible. We have developed our own software enabling analysis under GMP standards and have completed various validations. We have established a system capable of performing AUC under GMP standards.