AAV Vector Process Development
While drug discovery research targeting gene therapy using AAV vectors advances, the stable supply of AAV vectors remains a challenge. Developing technologies and establishing supply systems to stably manufacture high-quality AAV vectors in a short timeframe and at low cost is essential.
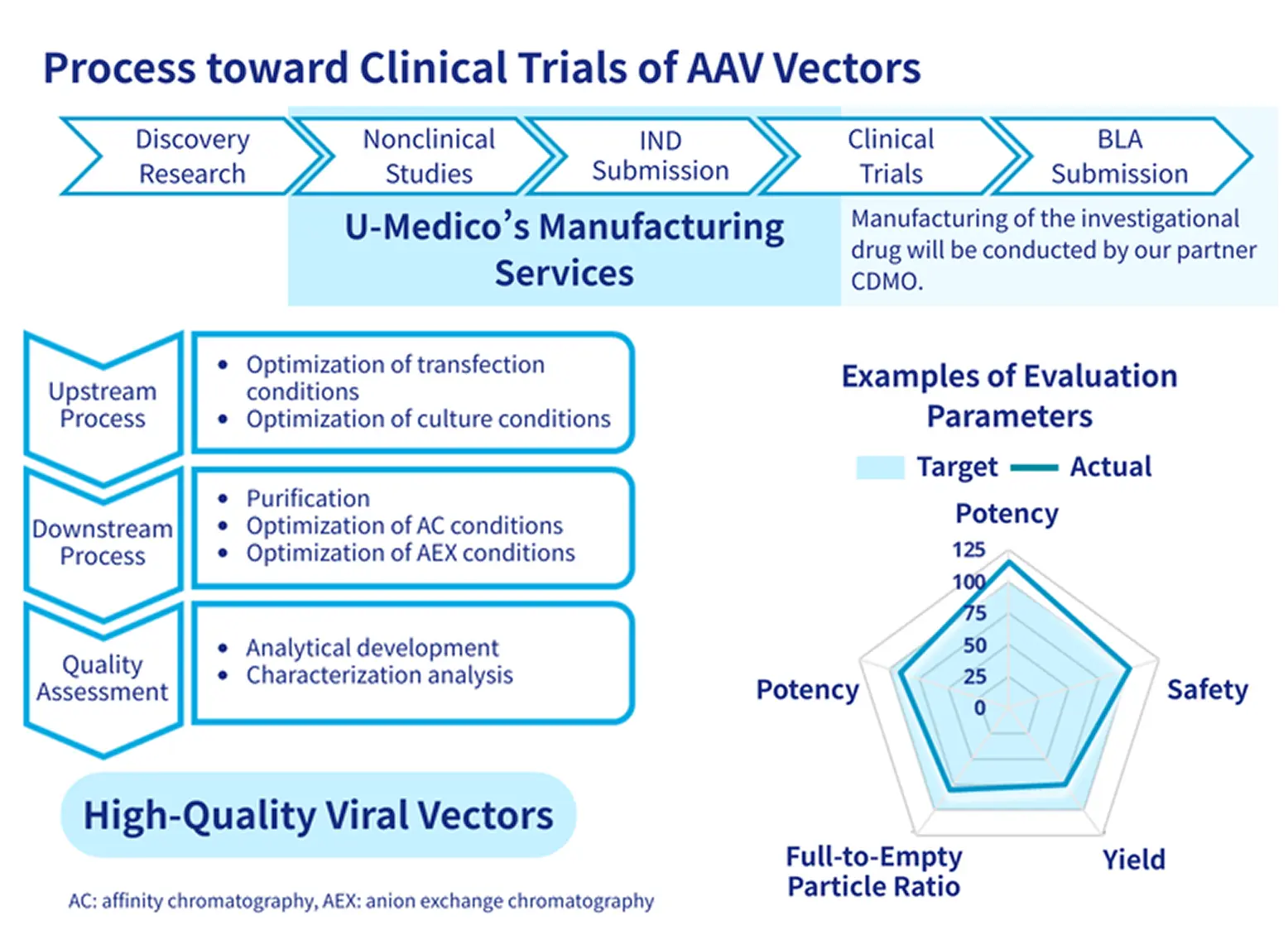
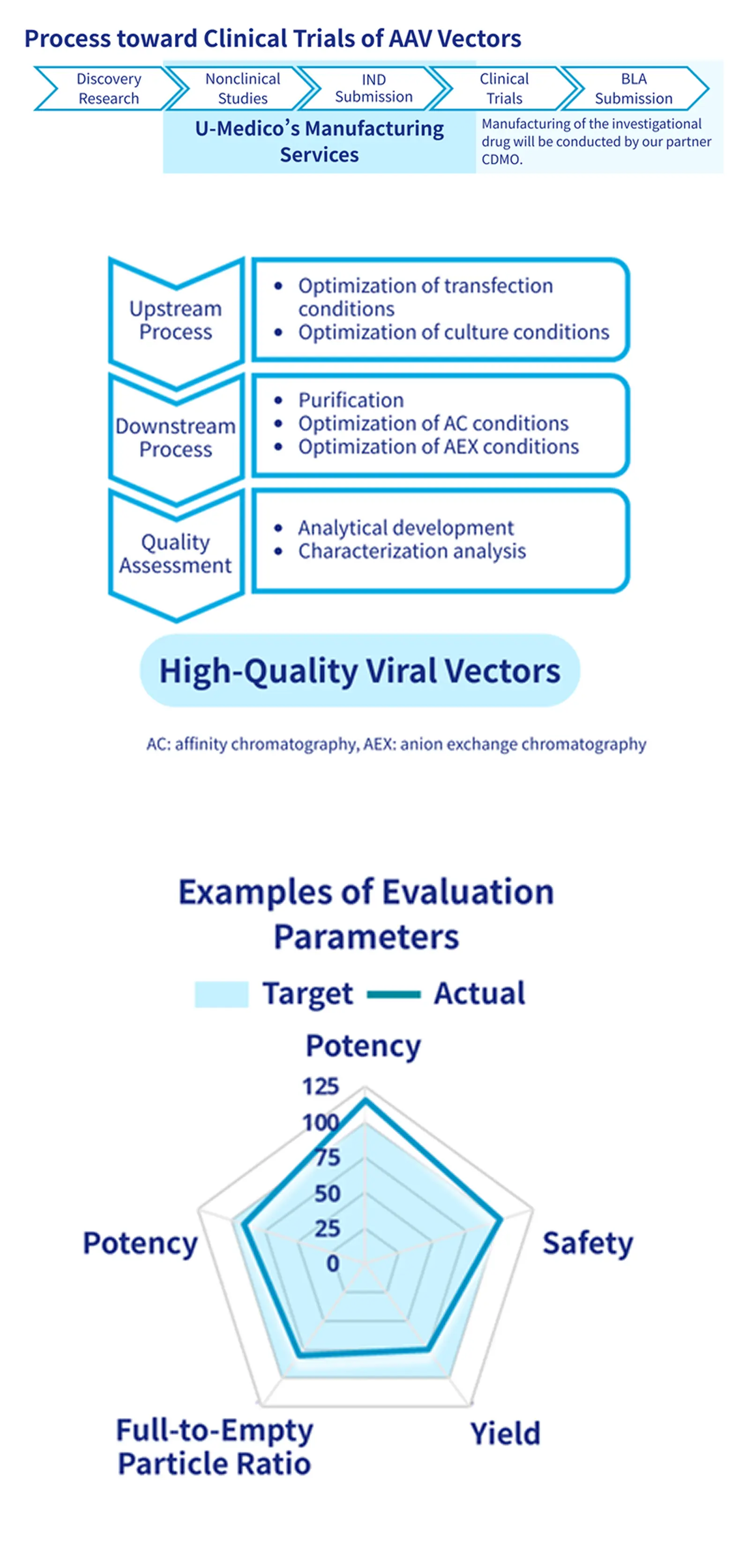
We support optimal process development tailored to each stage of AAV vector production. Through collaboration with GMP-capable CDMOs, we provide a comprehensive development framework from early research through clinical trials and commercial manufacturing to meet our clients' needs.
✓Flexible process development tailored to each stage
- Establish vector manufacturing processes suitable for each stage from product selection through nonclinical and clinical trials
- Supports scale-up from small-scale operations and establishes optimal manufacturing conditions
✓GMP-compliant manufacturing support
- Collaborate with GMP-compliant CDMOs to seamlessly support transition from nonclinical studies and clinical trials to commercial manufacturing.
- Achieving high-quality, high-yield production of AAV vectors
Entrust us with your process development to ensure stable supply and quality assurance of AAV vectors.

Chromatography System(AKTA pure)

CO2CO2 Incubator Shaker(New Brunswick S41i)
