This page introduces size distribution analytical service for viral vectors (VV) and oncolytic viruses (OV), etc. In the development of gene therapy drugs that use VV or OV, it is required to ensure that complete virus vectors (i.e., full particles) contain the desired nucleic acid. However, during the production of viral vectors, empty particles lacking nucleic acid and partial particles containing fragmented nucleic acids can also be generated. These particles may compete with full particles during the process of uptake by target cells via cell surface receptors, thereby reducing therapeutic efficacy or triggering unwanted immune responses. Therefore, it is critical to quantitatively assess the ratios of full, partial, and empty particles, which are considered Critical Quality Attributes (CQA). U-Medico not only provides a determination service of full/empty particle ratios but also offers comprehensive size distribution and composition analysis services that cover partial particles, including length information of fragmented nucleic acids, as shown below.
Molar extinction coefficient determination service of viral vector
The accurate determination of molar extinction coefficients plays a crucial role in the development and production of viral vectors. U-Medico offers a reliable method for determining the molar extinction coefficients of both full and empty viral vectors using analytical ultracentrifugation, which is described below. Once determined, the molar extinction coefficient can be utilized to accurately quantify the target component in routine spectroscopic analysis, including UV measurement and various chromatography techniques.
Full/empty ratio determination service
-
1.Analytical Ultracentrifugation (AUC)
Sedimentation velocity analytical ultracentrifugation (SV-AUC) is a powerful technique for quantitatively evaluating the dispersion state of samples, such as biotherapeutic drugs, including gene therapeutic drugs. This method involves continuously and directly observing the sedimentation behavior of the samples in a centrifugal force field, using external optics such as UV-visible, interference, or fluorescence optics, and analyzing the collected sedimentation velocity profile.
AUC provides high molecular size resolution and wide dynamic range, making it highly reliable for assessing molecular dispersibility in solution. Moreover, AUC is capable of quantifying both full and empty particles in F/E particle mixed samples, which can be challenging with other techniques like size exclusion chromatography and dynamic light scattering. Furthermore, AUC allows for the separation and quantification of partial particles and aggregates under various solvent conditions. For these reasons, AUC is the gold standard for physicochemical analysis in the quality control of viral vectors.
U-medico has published a scientific article reporting a quantitative evaluation of both full and empty particles in adeno-associated virus (AAV) samples using SV-AUC, which involves simultaneously probing absorbance and interference. In addition to the analytical ultracentrifuges XL-I and XL-A, the Optima AUC has been in operation since September 2022. By implementing the Optima AUC, we are now able to provide an analytical service that allows simultaneous measurement of multiple wavelengths, making it possible to completely identify the dispersion of AAV vector samples. We have also developed band sedimentation analytical ultracentrifugation (BS-AUC), which can determine the F/E particle ratio with reduced sample requirements.
Based on the know-how we have accumulated through the development of multiple analytical methods that can evaluate the dispersion state of AAV using AUC, we can propose and provide a world-class measurement service under the most suitable analytical conditions for your sample.
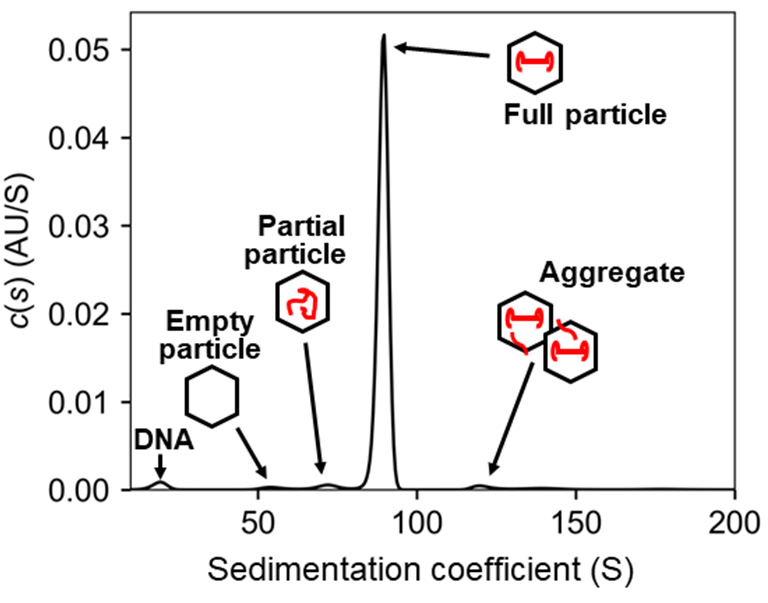
Comprehensive size distribution analysis of AAV using AUC

Optima AUC
-
2.Mass Photometry
The Mass Photometry (MP) method measures the molecular weight of biomolecules (nucleic acids, proteins, AAV vectors, aggregates, etc.) and nanoparticles in solution from the scattered light intensity of a single molecule on a glass substrate surface.
Mass photometry allows quantification using small sample volumes, as follows.
Sample Required Volume Antibody drugs A few nM, Less than 10 µL AAV vectors < 1012 vg/mL, 10 µL Using the MP method, we analyze the association state of antibodies, antibody-receptor interaction analysis (determination of dissociation constant KD), and the relative ratio of empty capsids (EC) and full particles (FP) of AAV vectors.
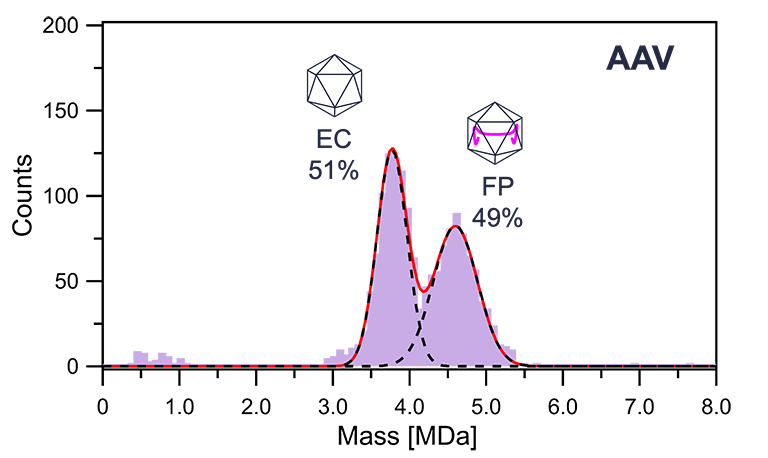
Examples of analyses

Refeyn OneMP
Although this technique requires only a small amount of sample and a short time to obtain results, it is not easy to obtain reproducibility and to interpret the results. Based on our experience, we provide high-quality analytical results.
