Biopharmaceuticals are subjected to various stresses during manufacturing, filling, transportation, storage, and administration. These stresses can potentially cause aggregates to form in solution. Aggregates are considered factors contributing to reduced activity and immunogenicity risks in biopharmaceuticals, necessitating their proper control and suppression.
Our company offers aggregate and particle analysis for quality testing, characterization, and stability testing of biopharmaceuticals and gene and cell therapies, utilizing optimal analytical methods tailored to particle sizes ranging from tens of nanometers to hundreds of micrometers.






Analytical Ultracentrifugation (AUC)
| Analysis Menu | Purpose |
|---|---|
| Sedimentation Rate Method | Sedimentation coefficient distribution, sample purity, aggregate quantification, stoichiometry of complexes, hydrodynamic shape, molecular weight and Stokes radius distribution, dissociation-association equilibrium constants, etc. |
| Sedimentation Equilibrium Method | Molecular weight, evaluation of intermolecular interactions based on the second virial coefficient, dissociation-association equilibrium constants, stoichiometry of complexes, etc. |
We also conduct testing under a quality assurance system to obtain the data required for pharmaceutical applications.
Mass Photometry
Mass Photometry (MP) is a method for measuring the molecular weight of biomolecules (nucleic acids, proteins, AAV vectors, aggregates, etc.) and nanoparticles in solution based on the scattered light intensity of samples (single molecules) on a glass substrate surface. It enables analysis of antibody association states, interaction analysis, and quantification of the full particle to empty particle ratio in AAV vectors. A key feature of the MP method is its ability to perform measurements using minute sample volumes of just tens of microliters.
We own two units, OneMP and SamuxMP, providing a wide range of measurement solutions.
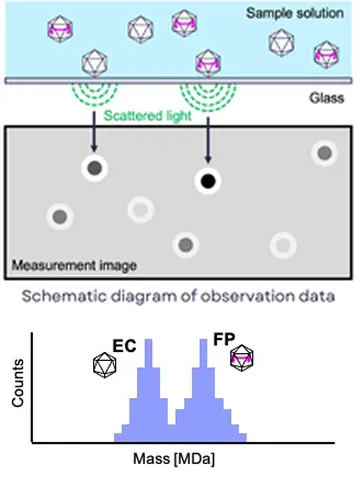
Principles of MP Method (Example: AAV Vector)
The MP method enables quantitative analysis using small sample volumes.
| Sample | Required Volume |
|---|---|
| Antibody drugs | A few nM, Less than 10 µL |
| AAV vectors | ~1012 vg/mL、10 µL |
Using the MP method, we evaluate the association state of antibodies and analyze antibody-receptor interactions (determining dissociation constants KD).
Additionally, it enables the rapid determination of the relative ratio of empty capsids (EC) to full particles (FP) in AAV vectors.
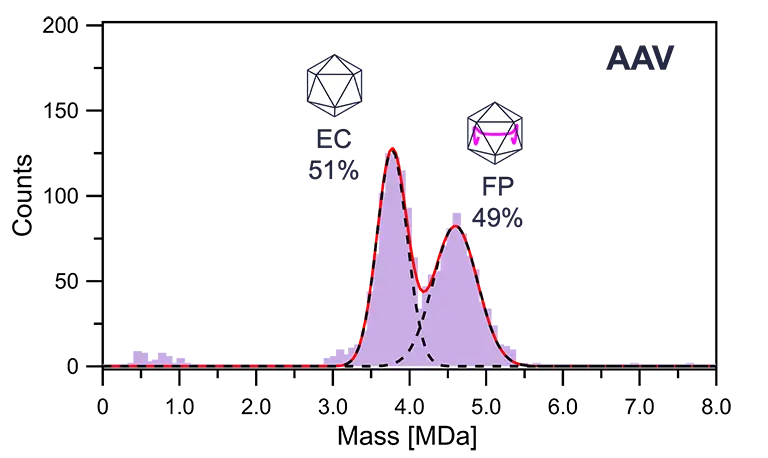
Examples of analyses

Mass Photometry(Refeyn OneMP)

Mass Photometry(Refeyn SamuxMP)
While this method uses small sample quantities and yields results quickly, achieving reproducibility and interpreting the results is not straightforward. Based on our extensive experience, we provide high-quality analytical results.
Quantitative Laser Diffraction (qLD) Method
Methods capable of quantitatively evaluating submicron-sized aggregates ranging from 0.1 to 1 µm are limited. However, because submicron-sized aggregates carry a high risk of inducing immunogenicity, their characterization is critically important. Aggregates Sizer is an effective method for quantitatively evaluating protein aggregates across a broad size range from submicron to micron size.
Flow imaging method
By continuously capturing high-resolution images of particles in a liquid flowing through a flow cell, information regarding particle size, number, and shape can be obtained.
Our company utilizes FlowCam for aggregate analysis in quality testing, characterization, and stability testing (long-term storage, accelerated, and stress testing) of biopharmaceuticals.
Additionally, using particle images acquired by FlowCam, we classify particles in pharmaceuticals into protein aggregates and silicone oil droplets through image analysis employing a convolutional neural network (CNN).
Silicone oil droplets released from silicone oil-coated medical devices into pharmaceuticals raise concerns about potential immunogenicity. Therefore, proper management is required.

Flow imaging device(FlowCam 8100)
